Metabolic Dysfunction-Associated Steatotic Liver Disease : Overview & Management
Introduction
Metabolic dysfunction-associated steatotic liver disease has emerged as a silent epidemic in modern medicine, affecting nearly 38% of adults worldwide and poised to become the leading cause of liver transplantation by 2030. Previously known as Metabolic dysfunction-Associated Steatotic Liver disease , this rebranding in 2023 better reflects its root cause — metabolic dysfunction tied to cardiometabolic risks like obesity, type 2 diabetes, and dyslipidemia. As interest in MASLD grows globally, this comprehensive guide explores its pathophysiology, diagnosis, management, and future directions, empowering healthcare professionals and patients with evidence-based insights from recent clinical guidelines and trials.pmc.ncbi.nlm.nih+3

What is Metabolic Dysfunction-Associated Steatotic Liver Disease?
MASLD describes excessive fat buildup in the liver (hepatic steatosis) in individuals without significant alcohol consumption, accompanied by at least one of five cardiometabolic criteria: overweight/obesity, type 2 diabetes, metabolic dysregulation (hypertension, dyslipidemia, or insulin resistance), or specific pediatric measures. This spectrum ranges from simple steatosis to metabolic dysfunction-associated steatohepatitis (MASH), characterized by inflammation and hepatocyte ballooning, which can progress to fibrosis, cirrhosis, and hepatocellular carcinoma.pmc.ncbi.nlm.nih+3
The nomenclature shift from MASLD to MASLD emphasizes metabolic drivers over alcohol exclusion, reducing stigma and improving diagnostic precision. Unlike alcoholic liver disease, MASLD links directly to systemic metabolic health, making it a harbinger of cardiovascular and renal complications.

The Pathophysiology of MASLD (Metabolic Dysfunction-Associated Steatotic Liver Disease)
At its core, MASLD arises from dysregulated lipid metabolism, where insulin resistance promotes de novo lipogenesis and impairs fatty acid oxidation in hepatocytes. This “multiple-hit” hypothesis evolves into lipotoxicity, triggering oxidative stress, endoplasmic reticulum stress, and inflammasome activation, which recruit immune cells and deposit extracellular matrix leading to fibrosis.
Genetic variants like PNPLA3 p.Ile148Met and TM6SF2 exacerbate lipid droplet accumulation and fibrosis progression, explaining inter-individual variability. The gut-liver axis contributes via dysbiosis, increasing endotoxin translocation and hepatic inflammation. Adipose tissue dysfunction releases free fatty acids and adipokines, amplifying systemic insulin resistance and completing the vicious cycle.pmc.ncbi.nlm.nih+2

Epidemiology and Public Health Impact Of MASLD
MASLD prevalence has surged to 30-40% in the general population, reaching 70% in type 2 diabetes cohorts, driven by obesity epidemics and sedentary lifestyles. In the U.S., it accounts for 25% of hepatocellular carcinoma cases without cirrhosis, while globally, it burdens healthcare with rising cirrhosis and decompensation rates.pubmed.ncbi.nlm.nih+2
Beyond the liver, MASLD elevates cardiovascular risk by 37%, manifesting as myocardial infarction, stroke, and heart failure through shared atherogenic pathways. Chronic kidney disease prevalence doubles in MASLD patients, with fibrosis stage correlating to eGFR decline. This multisystem impact underscores MASLD as a public health crisis demanding integrated cardiometabolic strategies.

Clinical Presentation and Diagnosis of MASLD
Most MASLD cases remain asymptomatic until advanced stages, detected incidentally via elevated ALT/AST or imaging for unrelated issues. Fatigue, right upper quadrant discomfort, or metabolic syndrome signs may prompt evaluation.
Diagnosis hinges on hepatic steatosis (≥5% on imaging/histology) plus cardiometabolic criteria, excluding secondary causes. Non-invasive tools include FIB-4/MASLD Fibrosis Score for fibrosis risk, vibration-controlled elastography (VCTE) for stiffness (>8 kPa flags advanced fibrosis), and MRI-PDFF for steatosis quantification. Liver biopsy confirms MASH but is reserved for diagnostic uncertainty or trial eligibility.pmc.ncbi.nlm.nih+2

Subtypes of MASLD and Cardiometabolic Risk Profiling
MASLD clusters into subtypes: PNPLA3-driven (genetic steatosis/fibrosis), insulin resistance-dominant, and adipose dysfunction phenotypes, each with distinct cardiovascular profiles. Adipose-centric subtypes show highest atherosclerosis risk via elevated atherogenic lipoproteins.
Cluster analysis via machine learning refines prognostication; insulin-resistant clusters predict diabetes progression, while genetic ones forecast rapid fibrosis. This subtyping informs tailored interventions, prioritizing CVD screening in high-risk groups.pmc.ncbi.nlm.nih+1

Management Strategies for MASLD
Lifestyle intervention remains first-line, targeting 7-10% weight loss to resolve steatosis in 50-90% of cases. Mediterranean or low-carb diets reduce hepatic fat by 30-40%, outperforming low-fat approaches. Aerobic exercise (150 min/week) plus resistance training improves insulin sensitivity independently of weight loss.pubmed.ncbi.nlm.nih+2
Comorbidity control is pivotal: statins for dyslipidemia (safe despite elevated enzymes), SGLT2i/GLP-1RA for diabetes, and antihypertensives per guidelines. Multidisciplinary teams coordinate care, with patient education enhancing adherence.
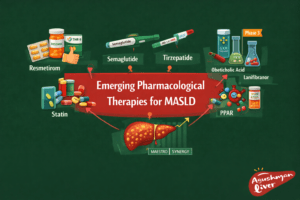
Emerging Pharmacological Therapies for MASLD
Resmetirom (THR-β agonist) earned FDA approval in 2024 for biopsy-proven MASH with F2-F3 fibrosis; MAESTRO-MASH trials showed 25-30% MASH resolution rates without fibrosis progression. Semaglutide (GLP-1RA) achieved 59% steatosis resolution in ESSENCE trial, with AASLD endorsing its use pending full approval.pmc.ncbi.nlm.nih+2
Tirzepatide (dual GLP-1/GIP agonist) reduced liver fat by 70% in SYNERGY-MASH, while pemafibrate (PPAR-α agonist) improved triglycerides and fibrosis markers. Obeticholic acid and lanifibranor await phase 3 outcomes, targeting FXR and PPAR pathways.pmc.ncbi.nlm.nih+2
| Therapy | Mechanism | Key Trial Results | Status pmc.ncbi.nlm.nih+2 |
| Resmetirom | THR-β agonist | 27% MASH resolution (F2-3) | FDA-approved (2024) |
| Semaglutide | GLP-1RA | 63% ≥30% fat reduction | Conditional AASLD guidance |
| Tirzepatide | GLP-1/GIP | 74% steatosis improvement | Phase 3 ongoing |
| Pemafibrate | PPAR-α | ↓ALT, fibrosis scores | Phase 3 |

Monitoring and Long-term Care of MASLD
Annual FIB-4/VCTE tracks progression; ELF score or MRE detects early fibrosis. Cardiovascular risk assessment via Framingham or ASCVD calculators guides statins/ASA use. HCC surveillance (ultrasound ± AFP) starts at cirrhosis diagnosis.pmc.ncbi.nlm.nih+2
Telehealth and apps support adherence; renal-hepatic clinics manage CKD overlap. Patient registries facilitate trial access and real-world data.pmc.ncbi.nlm.nih+1

Future Perspectives and Research Directions in MASLD
Precision medicine leverages polygenic risk scores for early intervention; multi-omics identifies biomarkers like miR-122 for MASH. Microbiome modulation via pre/probiotics or FMT shows preclinical promise.pmc.ncbi.nlm.nih+2
GLP-1RA expansions and pan-PPAR agonists headline pipelines; AI-driven subtyping optimizes trials. Public campaigns promote screening in metabolic clinics, curbing the MASLD tsunami.pubmed.ncbi.nlm.nih+1

Conclusion
MASLD demands proactive, holistic management blending lifestyle, pharmacotherapy, and surveillance to halt its progression and mitigate extrashepatic risks. Early detection via non-invasive tools and subtype-aware strategies promise better outcomes. Consult hepatologists for personalized plans—action today preserves liver health tomorrow.pmc.ncbi.nlm.nih+1
